First, the slope of a line can be determined by "Rise" over "Run" of the line on the graph.
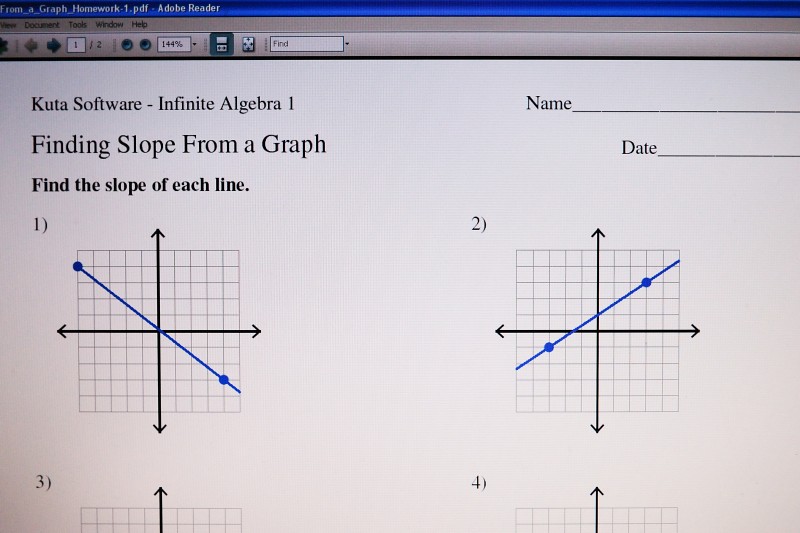
For graph one, to get from the dot on the far right to the dot on the far left, you travel up (rise) on the graph 7 spaces, then travel left (run) 9 spaces. Since we are traveling to the left, that's considered moving in the negative direction. The Slope is Rise over Run, or 7/-9.
Mathematical equation for the line is y=mx+b, where m is the slope and b is the y-intercept (where the line passes the y axis). So the equation for the line is y=-7/9x.
As for my favorite equation. I should have mentioned that it is an equation I learned in Chemistry, not math. A couple of you guessed a^2 + b^2 = c^2, or Pythagoras's Theorem. A very good equation!
But the most useful (and hence favorite) equation for me is:
PV = nRT
Or the Ideal Gas Law.
P = Pressure
V = Volume
n = moles or molecules of gas
R = a constant
T = Temperature
SO useful where I work! Because if the temperature goes up in a container and you have the same amount of gas in that container, guess what - the Pressure of the container will increase. This law is practically demonstrated if you blow up a balloon and stick it in the freezer - when you take it out, you will see that the balloon has shrunk in size. The volume shrinks because the temperature went down. As the gas heats up, the balloon will expand again.
OK, enough of the geeky stuff tonight! Back to our regular programming!
No comments:
Post a Comment